











$399.00
- Description
- Course Details
- Requirements
- Details
- Outline
- Learning Goals
- Behavioral Objectives
- Continuing Education State(s)
Description
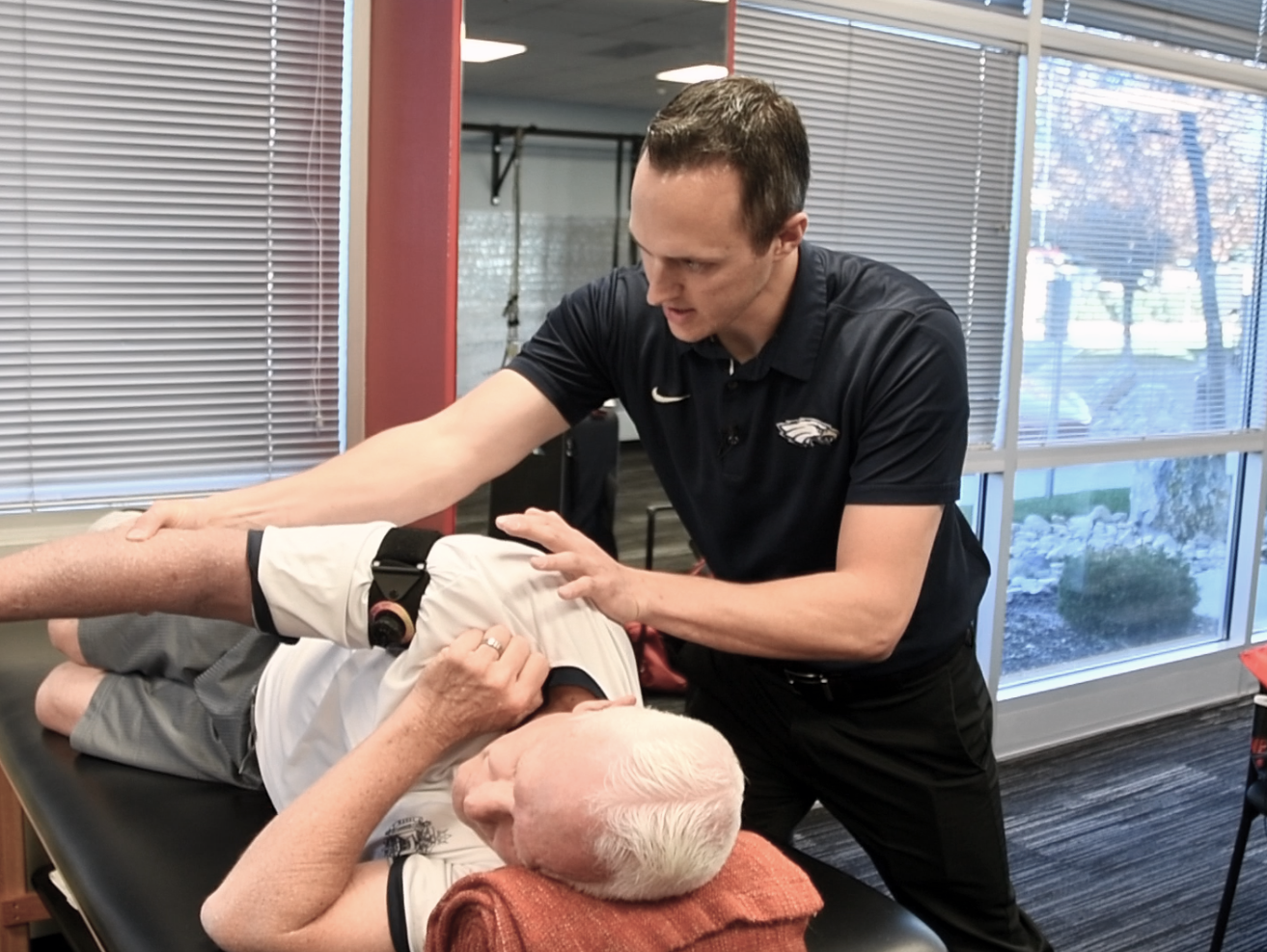
Take the lead on blood flow restriction when you learn from our experts.
The online course focuses on evidence-based research and proven clinical protocols and features an interactive app with regular updates.
Online library links to 500+ clinical studies and articles.
Course Details
| Instructor(s) | |
|---|---|
| Course Length | |
| Prerequisites | None |
| Platform |
Requirements
Personal Computer, Tablet, or Smartphone with access to Broadband Internet >1.4 mbps
Details
This evidence-based, equipment agnostic online course discusses the origins of blood flow restriction and identifies the metabolic mechanisms activated through the use of more than thirty case studies. The presentation combines lecture with presentation, audio, images, and videos.
Participants will learn not only the science behind BFRT, but also understand proper application for rehabilitation, performance enhancement, development of hypertrophy, and use in at-risk populations through demonstrations of actual BFRT protocols.
Drawn from research that includes more than 600 case studies and articles, the presentation discusses the contraindications, side effects, and instructs participants in how to screen potential patients and develop an Informed Consent document that focuses on the safe application of BFRT allowing participants to make informed decisions when choosing BFRT equipment and protocols.
FDA regulations and guidelines are addressed focusing on actual requirements of BFRT equipment. APTA, BOC and ACA definitions, requirements, and guidance are discussed. Participants will also receive instruction in identifying the quality of research in case studies using Sacketts Hierarchy as a guide.
Run Time: 9 hours 20 minutes (including Test)
Instructional Level: Physical Therapists, Physical Therapy Assistants, Athletic Trainers, Occupational Therapists, M.D.’s and Students
Examination: Yes
Requirements: Personal Computer, Tablet, or Smartphone with access to Broadband Internet (<1.4 mbps)
Course Evaluation: Yes
Certificate of Completion: Yes
Workbook/Handout: Yes
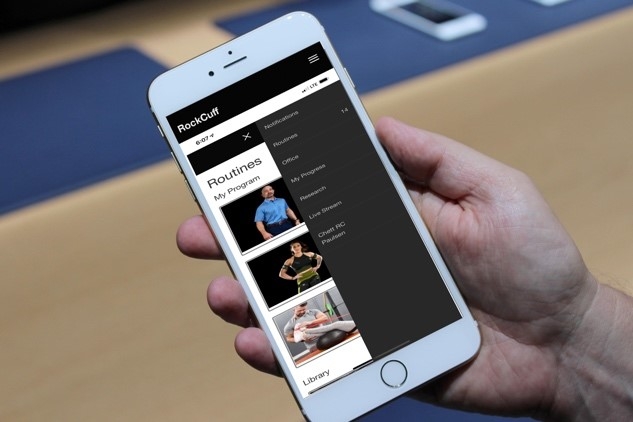
Course App:
The course includes an app downloadable via iTunes, and the Play Store. Also cloud-based access for personal computers
- Full motion demonstrations
- More than 45 minutes of protocol demonstrations from recognized experts in rehabilitation, fitness, and more
- Searchable online database with more than 1,000 Case Studies and Articles
- Discount code for the in-person course
- Discount code for BFR equipment
- Supplementation information on hydration, supplementation, nutrition, breathing, psychology
Outline
Length: 9 hours (including assessment quizzes)
Presentation Agenda:
120 min – Overview of Blood Flow Restriction (Nessler)
18 min – Patient Intake Considerations (Paulos)
56 min – Assessment Criterial and Application/Integration of BFRT (Paulos)
Knee
Shoulder
Elbow
63 min – Interactive Discussion on Various BFRT Topics (Levine and Nessler)
18 min – Application – LOP, RPF (Rate of Perceived Fatigue) (Nessler)
13 min – Cardiovascular Adaptations to Aerobic Training Stimuli (Levine)
29 min – Normal Muscle Reaction to Stimuli (Levine)
28 min – Device Selection Criteria and Options, Safety, Efficacy (Nessler)
40 min – Research Consideration (Levine)
29 min – Rehabilitation: BFRT plus Body Weight (Various)
30 min – Strength and Conditioning: BFRT and Body Weight (Various)
30 min – Fitness: BFRT and Body Weight –(Various)
30 min – Fitness: BFRT and Devices –(Various)
TRX Bands
Slam Ball
Battle Rope
Tire
26 min – Rehabilitation Case Study (Paulos)
Learning Goals
Upon completion of the course, the participant will be able to:
- Compare the benefits of BFRT to the use of high-intensity interval training (HIIT) for a patient with limited mobility following ACL surgery.
- Propose an integrated return-to-play protocol with at least 4 BFRT exercises for a post-surgical patient following ACL surgery using BFR.
- Theorize the expected outcomes when using BFR protocols for elderly patients with muscle disuse.
- Design a strength and conditioning protocol using 4 BFRT exercises for female volleyball players using BFRT.
- Propose 3 changes using BFRT to an existing non-BFRT rehabilitation protocol for a patient with shoulder pain by supplementing with BFRT.
- Using case study evidence, evaluate measured CSA and strength gains for a patient in their early 20’s based on chemical mechanisms produced by BFRT.
- Design a comprehensive patient/athlete intake questionnaire as part of an Informed Consent form based on evidence and local board requirements.
Behavioral Objectives
Upon completion of the course the participant will be able to:
- Design a comprehensive intake questionnaire as part of an Informed Consent form based on evidence and local board requirements.
- Able to apply case study evidence, evaluate measured CSA and strength gains for a patient in their early 20”s based on the chemical mechanisms produced by BFRT
- Compare the benefits of BFRT to the use of low-intensity resistance training LIR for a patient with limited mobility following ACL surgery
- Theorize the expected outcomes when using BFR protocols for elderly patients with muscle disuse
- Propose and integrate return-to-play protocol utilizing at least 4 BFRT exercises for a post-surgical athlete following ACL surgery
- Design a strength and conditioning protocol using 4 BFRT exercises for female volleyball players to improve vertical leap
- Propose 3 changes using BFRT to an existing non-BFRT rehabilitation protocol for a patient with shoulder pain
- Design a fitness program using body-weight and BFRT for a person who travels extensively
- Develop an integrated strength and conditioning program for an elitie athlete to improve VO2 Max and VE Max
- Propose a change to your current return-to-play protocol for an injured athlete sidelined after a rotator cuff injury using BFRT that will reduce atrophy that accompanies inactivity from limited use.
Continuing Education State(s)
Alaska, Arizona, Arkansas, California, Colorado, Delaware, District of Columbia, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Kansas, Kentucky, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, North Carolina, North Dakota, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Utah, Vermont, Virginia, Wisconsin, Wyoming, Puerto Rico

